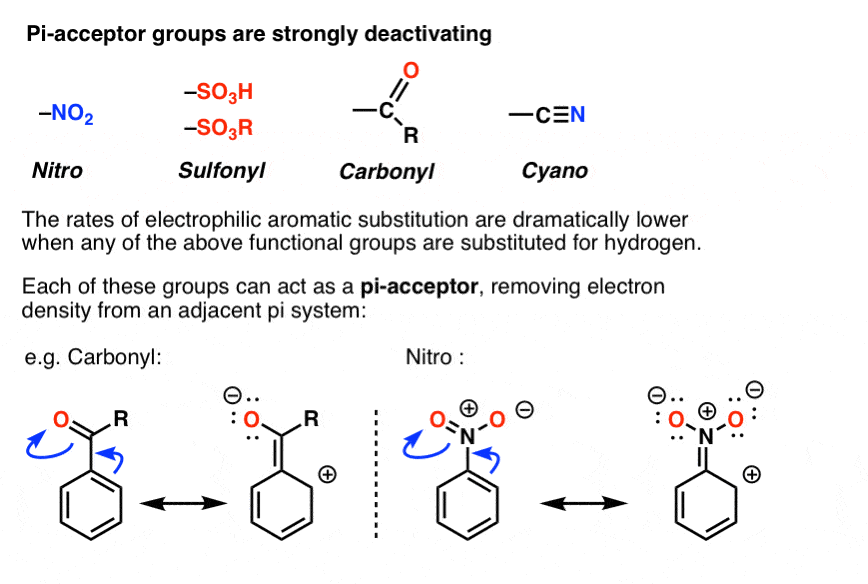
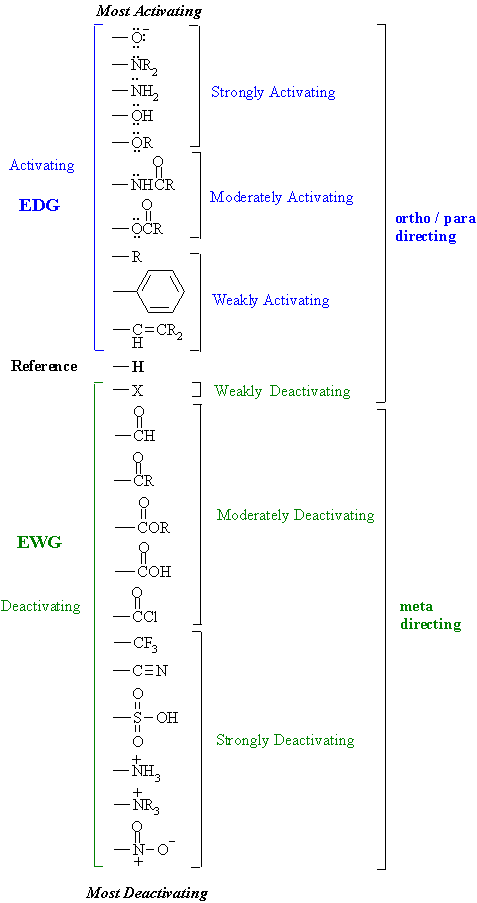
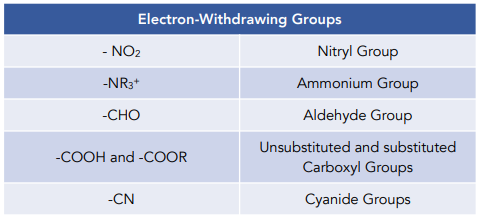
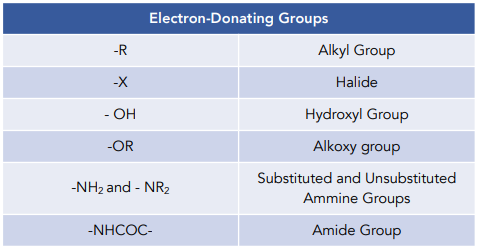
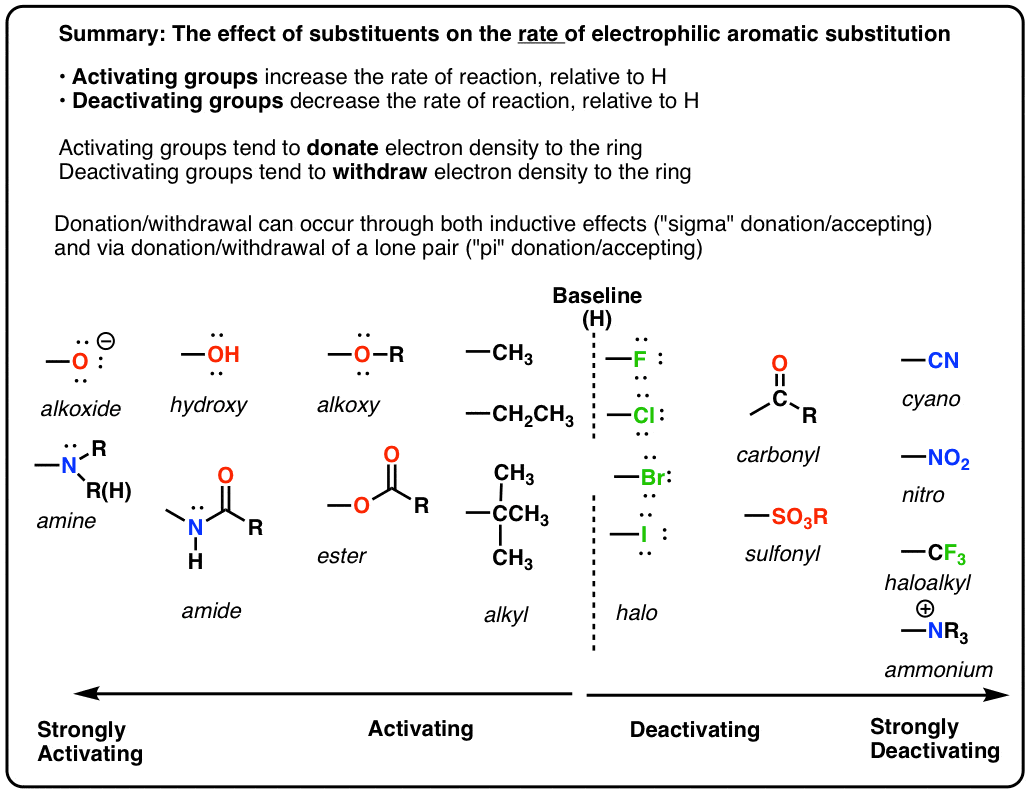
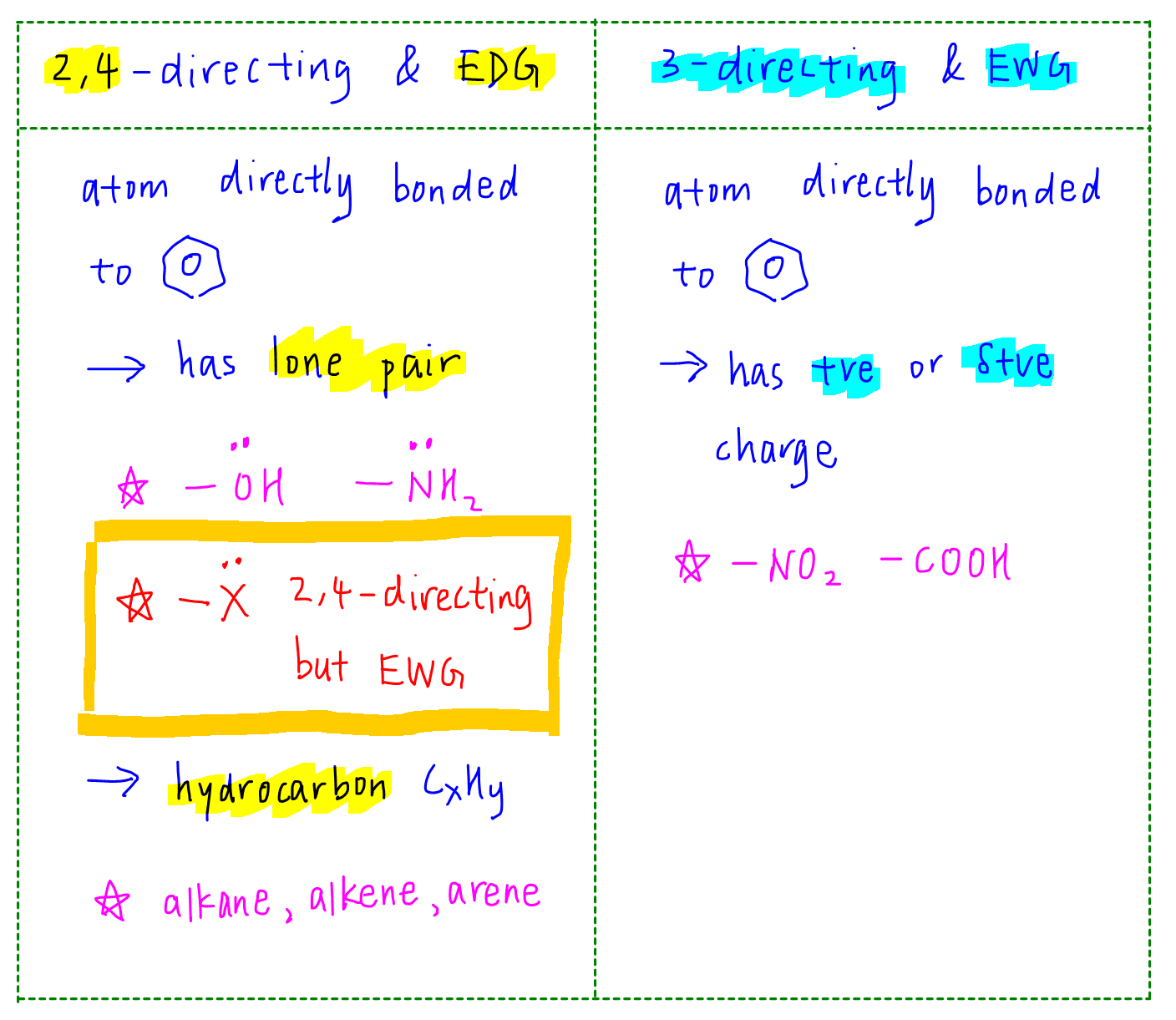
SOLVED: Electron-Withdrawing Groups Carbonyls When one of the atoms directly connected to the carbonyl carbon isa heteroatomas in esters and amides lone Pairh from the heteroatom can bepushed into the carbonyl group
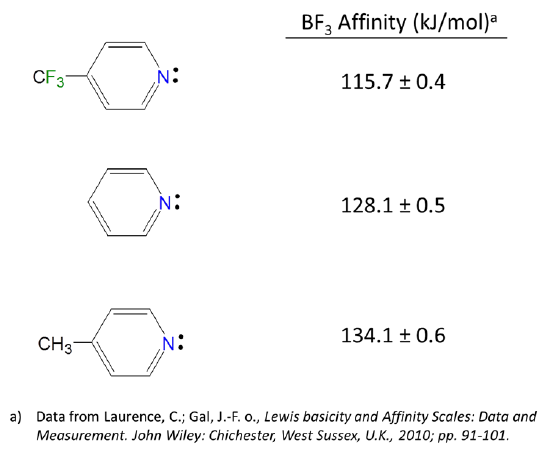
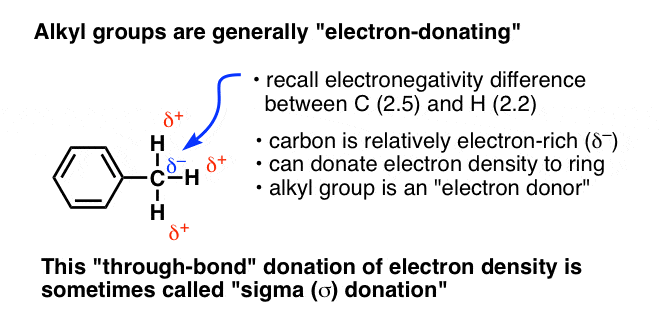
6.4.2: All other things being equal, electron withdrawing groups tend to make Lewis acids stronger and bases weaker while electron donating groups tend to make Lewis bases stronger and acids weaker -

Enhancing effects of electron-withdrawing groups and metallic ions on halogen bonding in the YC6F4X···C2H8N2 (X = Cl, Br, I; Y = F, CN, NO2, LiNC+, NaNC+) complex. | Semantic Scholar

organic chemistry - Amine group - electron donating or withdrawing group? - Chemistry Stack Exchange
Is it true that fluorine is always the strongest electron withdrawing group (EWG) due to fluorine's unrivaled electronegativity? - Quora

In the context of this question, are amines classified as electron donating because of their sp2 hybridization due to resonance? Because afaik, amines are electronegative and therefore electron withdrawing groups. : r/Mcat

Substituent Effects of Tetracoordinate Boron in Organic Synthesis - Taniguchi - 2022 - Chemistry – A European Journal - Wiley Online Library
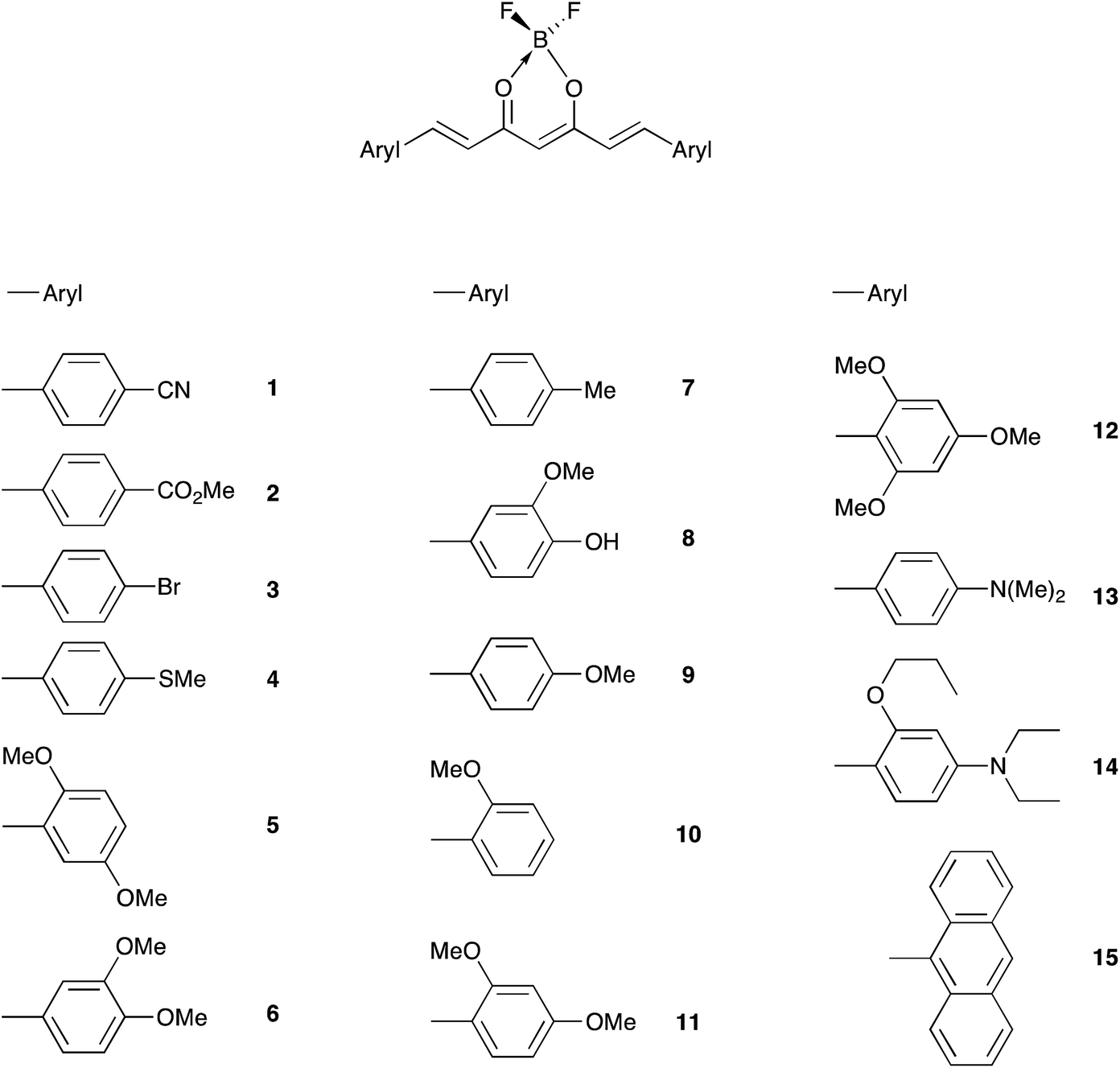
Influence of the electron donor groups on the optical and electrochemical properties of borondifluoride complexes of curcuminoid derivatives: a joint ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA25436E
![Study the following sequence of reactions and identify the product (Y) . CH3CHO + HCHO [heat]dil.NaOH X [H3O^ + ]HCN Y Study the following sequence of reactions and identify the product (Y) . CH3CHO + HCHO [heat]dil.NaOH X [H3O^ + ]HCN Y](https://toppr-doubts-media.s3.amazonaws.com/images/2140650/bcc67e75-4fb0-4420-aaef-47cd4d3dbd3e.jpg)
Study the following sequence of reactions and identify the product (Y) . CH3CHO + HCHO [heat]dil.NaOH X [H3O^ + ]HCN Y