SOLVED:From the value of Kf listed in Table 17.1, calculate the concentration of Ni^2+ in 1.0 L of a solution that contains a total of 1 ×10^-3 mol of nickel(II) ion and

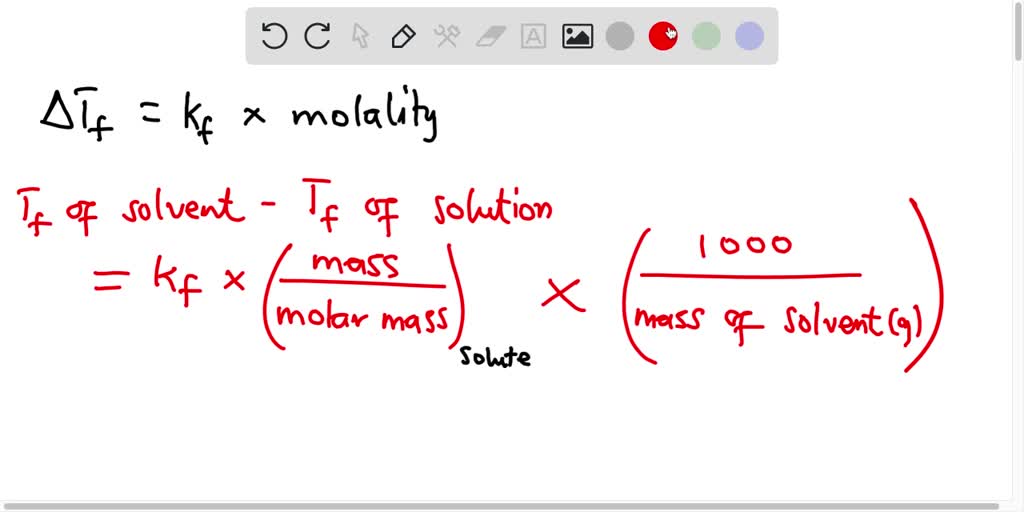
Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (kf for water = 1.86 K kg mol^-1)

How are Kf values relevant in calculations of the melting temperature of a solution? | Homework.Study.com
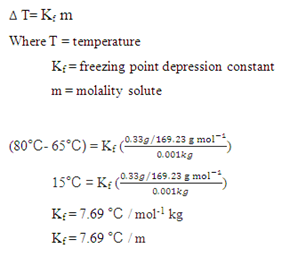
Q6 Calculate the freezing point of an aqueous solution containing 10.50 g of MaBrz in 200 gof water (molar mass of MgBr2 184 g mol 1 ). Given Kf for water = 1.86 KKg mor

Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol^-1 ) in 250 g of water. ( Kf of water = 1.86 K kg mol^-1 ).
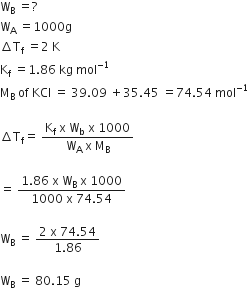
Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (Kf for water = 1.86 K kg mol-1) from